
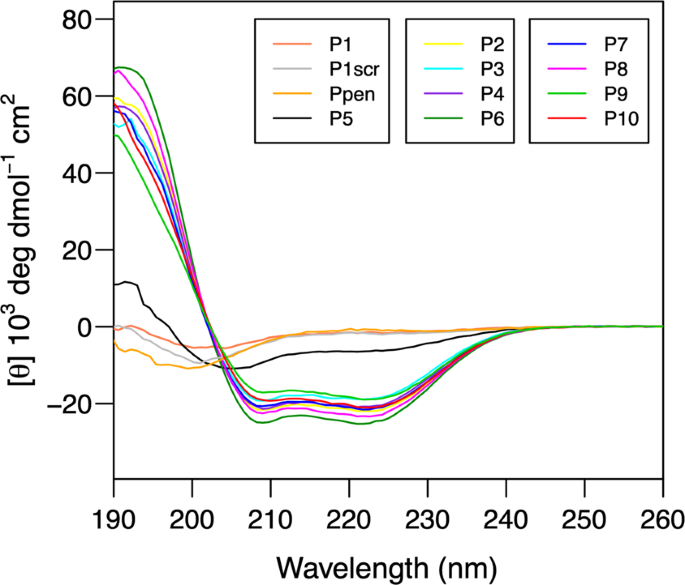
Based on the structure, we show that some reported loss-of-binding mutations involve the PD-1/PD-L1 interaction but that others compromise protein folding. Mapping conserved residues allowed the identification of residues that are important in forming the PD-1/PD-L1 interface. This places the loops at the ends of the IgV domains on the same side of the PD-1/PD-L1 complex, forming a surface that is similar to the antigen-binding surface of antibodies and T cell receptors.
PD-1 and PD-L1 interact through the conserved front and side of their Ig variable (IgV) domains, as do the IgV domains of antibodies and T cell receptors. Here we present the crystal structure of murine PD-1 in complex with human PD-L1. Release from PD-1 inhibitory signaling revives “exhausted” virus-specific T cells in chronic viral infections. Signaling through the programmed death 1 (PD-1) inhibitory receptor upon binding its ligand, PD-L1, suppresses immune responses against autoantigens and tumors and plays an important role in the maintenance of peripheral immune tolerance.


 0 kommentar(er)
0 kommentar(er)
